Abstract
Introduction: The overlap in the median age at diagnosis for patients with plasma cell myeloma (PCM, 69 years) and clonal hematopoiesis (CH, incidence peaking over 60 years), results in a natural risk of co-occurrence of these two conditions (Cowan et al JAMA 2022, Jaiswal et al N Engl J Med 2014). The incidence of CH in patients with plasma cell myeloma is 22%-33%, with the most common variants found in DNMT3A, TET2, TP53, ASXL1 and PPM1D (Mouhieddiene et al Nat Commun 2020, Matarraz et al Haematologica 2012). Combination therapy with proteasome inhibitors, immunomodulatory drugs, steroids and autologous stem cell transplantation (AUTO) is associated with a median overall survival up to 10.5 years (Cowan et al JAMA 2022), with therapy-related myeloid neoplasm (T-MN) reported in 3.3% patients with PCM (Mouhieddiene et al Nat Commun 2020). For poor-risk PCM, tandem AUTO followed by non-myeloablative allogeneic stem cell transplantation (ALLO-HCT) has been explored, with median OS of 6-7 years post ALLO-HCT reported (Gahrton et al J Clin Med 2020, Luoma et al Ann Hematol 2021). The efficiency of ALLO-HCT in being able to sustainably eradicate host CH and the resulting impact on the risk of T-MN is not known.
Methods: We retrospectively identified patients with PCM receiving tandem AUTO followed by ALLO-HCT from 2010-2020 treated at the Alfred Hospital, Melbourne, Australia. DNA from peripheral blood sorted for CD3+ lymphocytes was examined at two time points, immediately prior to ALLO-HCT and after, when peak donor chimerism was achieved. CD3+ cells were isolated using specific RosetteSep™ enrichment cocktails and assessed by flow cytometry. DNA preparation was done using the Qiagen QIAamp DNA Blood Mini Kit. Next generation sequencing (NGS) was performed using the Roche KAPA HyperCap workflow with a custom 48-gene panel. Fastq files were run through the Illumina Dragen Enrichment analysis pipeline and variants were curated using Agilent Alissa Interpret. Variants with a minimum variant allele frequency (VAF) of 1% were included. The cut-off date for clinical follow up was 1st March 2022. The study was approved by Alfred Health (621/21). Descriptive statistics was used for baseline characteristics and the Kaplan-Meier method for overall survival.
Results: A total of 89 patients with PCM received the tandem AUTO-ALLO HCT procedure between 2010-2020. Paired pre- and post- ALLO HCT DNA samples were available for 62 patients. Median age was 56 years (range 23-68), median prior lines of myeloma therapy was 4 (2-13), including 30 (48%) patients who had received >1 prior AUTO pre ALLO-HCT, 30/62 (48%) patients received AUTO-ALLO HCT for high risk PCM (based on combination of diagnostic karyotype, ISS stage, response to initial treatment or extramedullary/plasma cell leukaemia) and 32/62 (52%) for relapse management after initial AUTO.
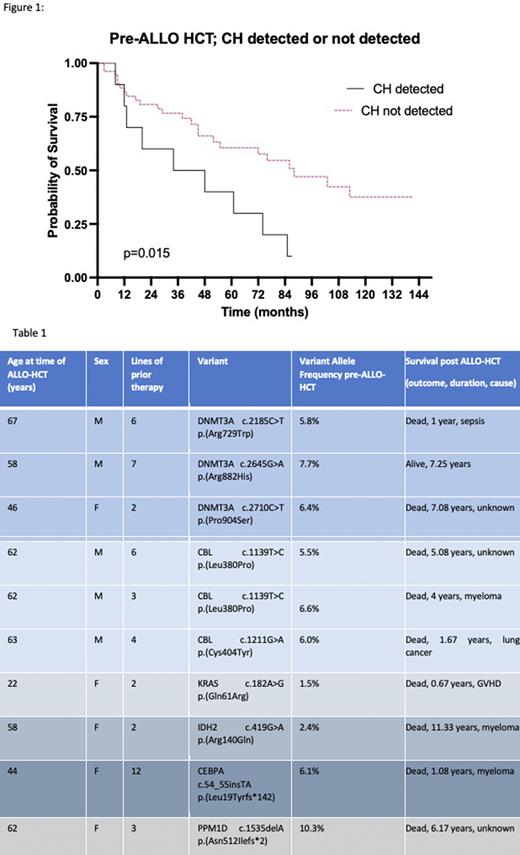
At the pre-ALLO-HCT timepoint, 10/62 (16.1%) patients had pathogenic variants detected in the CD3+ fraction of peripheral blood (Table 1). The median VAF was 6.1% (range 1.5-10.3%). No patient had more than one variant identified. By the data cut-off, 33/62 (53%) patients had died. Median OS was 6.33 years (0.25-9.42). No patients had so far developed T-MN, after a median follow up time of 7.25 years (2.08-11.75). Median OS was shorter for patients with CH detected, compared to patients without detectable CH pre-ALLO-HCT (3.41 vs 7.33 years; p=0.016, Figure 1). Of the deaths, 14/33 (42%) were due to myeloma, 2/33 (6%) sepsis, 3/33 (9%) sepsis and graft versus host disease (GVHD), 3/33(9%) GVHD alone, 1/33 (3%) fall, 1/33 (3%) lung cancer and 9/33 (27%) unknown. Death in patients with CH detected is displayed in Table 1.
Conclusions: In CD3+ sorted peripheral blood of patients with PCM, the frequency of detected CH was 16.1%, higher than expected in the healthy population. No TP53 variants and only one PPM1D variant was identified in this heavily pre-treated population. After ALLO-HCT, no cases of T-MN were reported, with a median follow-up interval of 7 years. We hypothesize that CH will be efficiently and sustainably eradicated in patients receiving AUTO-ALLO HCT and that this may reduce the longer-term risk of developing T-MN. The lower OS in patients with CH identified pre-ALLO-HCT was unexpected. A full analysis of pre- and post-ALLO-HCT blood DNA variants will be presented.
Disclosures
Anstee:Walter and Eliza Hall Institute: Patents & Royalties: Dr Anstee is an employee of the Walter and Eliza Hall Institute and is eligible for a fraction of the royalty stream related to Venetoclax. Tiong:Servier: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Amgen: Speakers Bureau. Spencer:Amgen: Consultancy, Honoraria; Haemalogix: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria. Wei:Macrogenics: Honoraria; Walter and Eliza Hall Institute of Medical Research: Patents & Royalties; BeiGene: Consultancy; Astex: Research Funding; Syndax: Research Funding; Astellas: Honoraria, Speakers Bureau; Gilead: Honoraria; Shoreline: Honoraria; Agios: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Research Funding; Pfizer: Honoraria; Roche: Honoraria; AstraZeneca: Honoraria, Research Funding; Servier: Consultancy, Honoraria, Other: Travel, Accommodations, and Expenses, Patents & Royalties: Filing Date: 21/07/2017. Combination of a BCL-2 inhibitor and a MCL-1 inhibitor, uses and pharmaceutical compositions thereof. A. Wei, D. Moujalled, G. Pomilio, A.L. Maragno, O. Geneste, A. Claperon, H. Maacke, E. Halilovic, D. Porter, E. Morris, Y. Wang, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, and Expenses, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal